Histopathology & Drug Development

Histopathology & Drug Development – HE Stain Guide Stop Frozen Sections From Shattering – Dr. Song’s 30-Second Fix Fix Brittle FFPE Sections Like a Pro! Digital Transformation and AI in Histopathology
iCura Diagnostics’ Commitment to Developing Reliable, Affordable, and Easy-to-Use Covid-19 Diagnostic Kits
We are intensively working on development of Covid-19 diagnostic kits to provide reliable, affordable, and easy-to-use Covid-19 rapid detection methods to face the current challenges in our communities. At iCura Diagnostics, we are dedicated to creating innovative Covid-19 diagnostic kits to address the challenges of the current pandemic. Our mission is to provide reliable, affordable, […]
Advancing Clinical Trials and Medical Diagnoses with FDA-Approved Circulating Tumor Cell (CTC) Services

Advancing Clinical Trials and Medical Diagnoses with FDA-Approved Circulating Tumor Cell (CTC) Services iCura Diagnostics takes great pride in offering state-of-the-art Circulating Tumor Cell (CTC) Services, built on the FDA-approved platform. Our CTC services are specifically designed to deliver cutting-edge capabilities for the detection and analysis of circulating tumor cells. With a strong focus on […]
Introducing Our Validated 6-Plex 7-Color Lung Cancer and Melanoma Panel: Precision Diagnostics Redefined
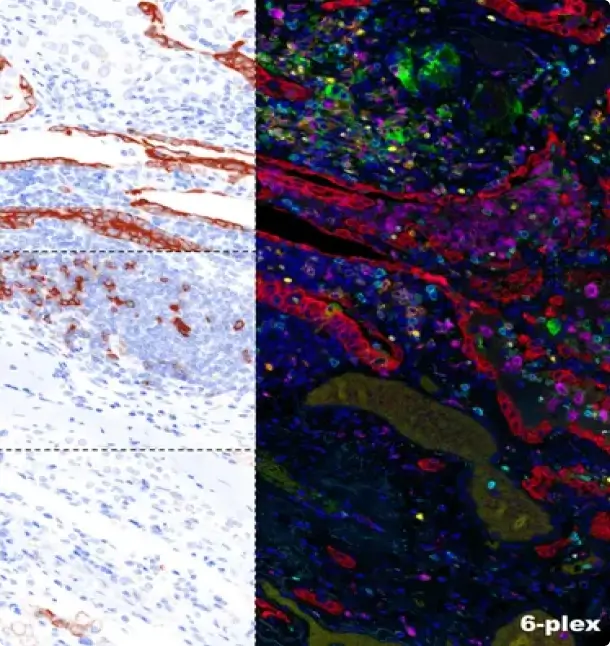
Introducing Our Validated 6-Plex 7-Color Lung Cancer and Melanoma Panel: Precision Diagnostics Redefined At iCura Diagnostics, we are thrilled to announce the successful validation of our groundbreaking 6-Plex 7-Color Lung Cancer and Melanoma Panel. This cutting-edge panel revolutionizes precision diagnostics, offering a comprehensive and accurate approach to detect and monitor lung cancer and melanoma. Designed […]
Elevating Oncologic Studies with State-of-the-Art Next Generation Sequencing (NGS) and Digital PCR Platforms

iCura Diagnostics is proud to offer advanced Next Generation Sequencing (NGS) and Digital PCR platforms that provide high throughput and reliable genetic testing for oncologic studies. With the capacity to process both tumor tissue and liquid biopsies, our state-of-the-art technologies deliver valuable insights into cancer genetics, enabling personalized medicine and improved patient outcomes. NGS, a […]
Transforming Clinical Trial Studies with iCura’s Validated Digital Pathology Services: Multiplexing IF/IHC, Image Analysis, and More

Our full line of Digital Pathology Services is built on industry-leading digital pathology solution platforms that are fully validated for Clinical Trial Studies. We perform Whole Slide Imaging, Multiplex IF/IHC, and Image Analysis services under CLIA and GLP compliance. iCura Diagnostics takes pride in offering a comprehensive range of Digital Pathology Services that are designed […]
iCura Diagnostics Certified as Fully Qualified Service Provider Partner by AKOYA Biosciences
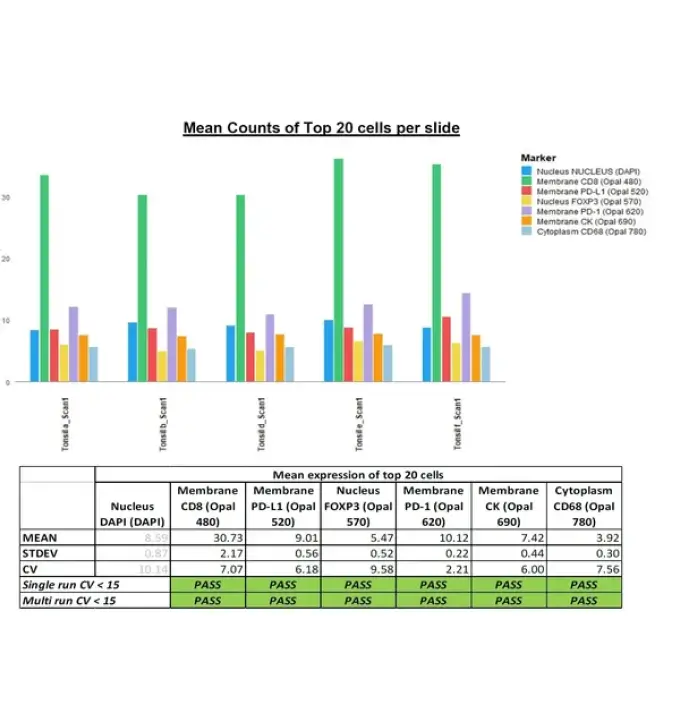
iCura Diagnostics Certified as Fully Qualified Service Provider Partner by AKOYA Biosciences We are excited to announce that iCura has been recognized as AKOYA Biosciences‘ first-ever Fully Qualified Service Partner. This distinction showcases our exceptional quality and commitment to delivering outstanding services in the field of contract research organizations (CROs). We are proud to be at […]
